Water Properties
Table of Contents
What is the Specific Heat of Water
The specific heat of water is 4182 J/kg°C, which is a high specific heat capacity and is sometimes taken as 4,200 J/kg °C for ease in calculations. Specific heat capacity is the defined as the amount of heat per unit required to raise the temperature by one degree Celsius.
This means it takes water roughly 4200 J (Joules) to raise its temperature of 1 kg water by 1 °C. Imagine a 5m x 5m x 2m water tank with a volume of 75m3, at a water density of 1000 kg/m3, which gives us a weight of 75,000 kg of water. Just from looking at the sheer weight of water, we can easily tell that it will take a lot of energy to increase the temperature by 1°C. This is an important feature of water, which is discussed further down the article.
The SI units for specific heat are kJ/kg°C or J/kg °C.
What is Specific Heat and why is it important?
As mentioned previously, specific heat is the amount of heat required to make an object/substance 1 degree hotter/warmer. There are some materials which require a lot of heat to become warmer by 1 degree °C (Water) and others that do not require that much heat.
When materials are heated, the molecules gain kinetic energy and becomes hotter (molecules start moving faster) and different materials require a different amount of energy which depend on the mass of the material and heat capacity of the material.
An example of materials with lower capacities are Gold, Steel, and Wood when compared to water. Therefore, gold and steel will warm up faster and cool down faster in comparison to water, without requiring too much energy.
This is easily seen when heating steel when cars are left out on the drive. On a hot day, the temperature of the car body will heat up rapidly to a high temperature, which is hot enough to burn people and then rapidly cool down at night. However, when we consider a pool of water, we notice that it will not rapidly heat up and start boiling or quickly cool down after we have made a cup of tea or coffee. This is due to the fact that the specific heat of water is high in comparison to other materials.
This is important of water, being able to maintain its temperature throughout the day and night. Otherwise, this would be disastrous for wildlife and fish in rivers and oceans, as the local ecosystem and environment would not be able to survive and grow efficiently if there were constant changes in the water temperature.
The equation for specific heat is
\(Q = cm \Delta T\)
where Q = heat added
c = specific heat
m = mass
\(\Delta T\) = change in temperature
Specific Heat Table
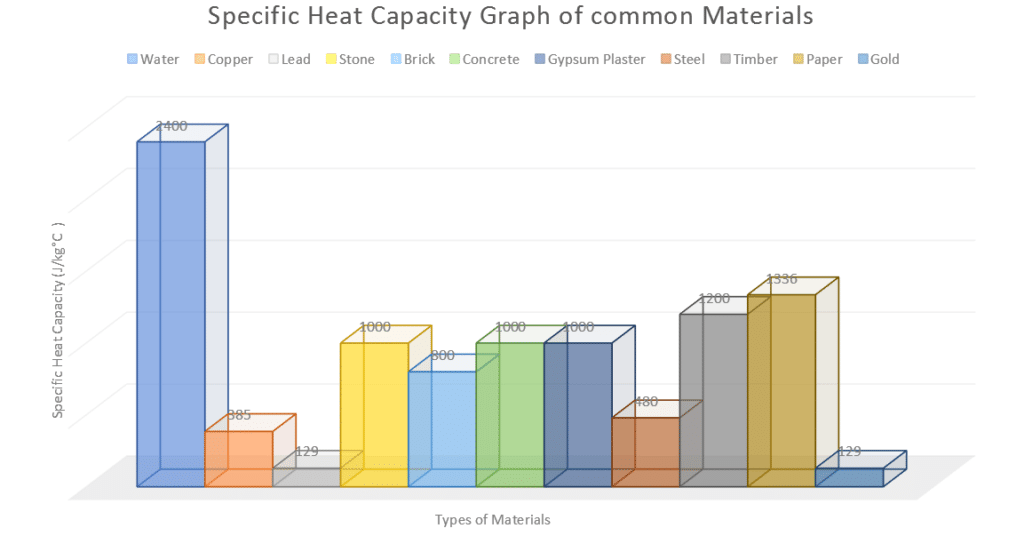
The table below is a list of materials with different specific heat capacities.
It difference between the specific heat capacity of water and the other materials is apparent and quite large, which gives water its unique properties.
It is understood that atomic composition of water and its strong bonds between the hydrogen and oxygen atoms is why a lot of energy is needed to heat water in comparison to other materials.
The reason that the bonds are so strong is due to hydrogen bonding (molecules that contain OH,NH atoms in their structure). Once the water becomes heated, the heat is used to loosen the hydrogen bonds and not increase the water’s kinetic energy. This is just a brief description of the breaking down of bonds and there is a much more detailed description is scientific journals and even Wikipedia.
This is thought to be the same reason as to why liquid ammonia has a higher specific heat capacity when compared with the specific heat of water.
The table below shows the specific heat of water as 2400 J/kg°C and the lowest is 129 J/kg°C with Lead and Gold. This indicates both those materials can heat up quickly and cool down quickly.
| Material | Specific Heat Capacity in J/kg°C |
| Water | 2400 |
| Copper | 385 |
| Lead | 129 |
| Stone | 1000 |
| Brick | 800 |
| Concrete | 1000 |
| Gypsum Plaster | 1000 |
| Steel | 480 |
| Timber | 1200 |
| Paper | 1336 |
| Gold | 129 |
Summary
- The specific heat of water is 4182 J/kg°C exactly.
- Water has a high specific heat when compared with other materials.
- Water molecules contain strong bonding between the hydrogen and oxygen atoms which means lots of energy (heat) is required to increase the temperature (water boils at 100 °C)
- Lead and Gold have very low specific heat capacities
- Heat Capacity does not refer to the amount of heat that can be absorbed or boiling point, but it is the amount of heat required to increase the temperature by 1 °C
